The All India Institute of Medical Sciences (AIIMS) Ethics Committee has approved the human clinical trials of India’s very first coronavirus vaccine candidate ‘Covaxin’. Covaxin is developed by Bharat Biotech, a Hydeberad-based firm in collaboration with the Indian Council of Medical Research (ICMR) and the National Institute of Virology (NIV).
As the coronavirus continues to ravage the health of millions of people globally, scientists and medical experts are continuously working to develop a vaccine for the highly infectious contagion. It should be mentioned that more than 155 vaccine candidates globally have entered various stages of trials and 23 out of these, have already started human trials.
Dr Sanjay Rai, Principal Investigator of the Covid-19 Vaccine trial at AIIMS commenced the whole process earlier this week. He also informed that the first dose of vaccine is likely to be administered to volunteers on Thursday.
AIIMS is one of the 12 medical institutes that has been selected for phase 1 and 2 of human trials by the Indian Council for Medical Research (ICMR). The 12 sites include AIIMS Patna and Delhi, SRM hospital in Chennai, Rohtak’s Post-Graduate Institute of Medical Sciences and Hyderabad’s Nizams Institute of Medical Sciences among others.

DCGI has approved two vaccines for Phase 1 and Phase 2 trials
The Drug Controller General of India, Central Drugs Standard Control Organisation (CDSCO), and the Ministry of Health & Family Welfare, granted permission to initiate Phase-I and II human clinical trials. The DCGI has permitted two vaccines, the first by Bharat Biotech International Limited in collaboration with the ICMR, and the second by Zydas Cadila Healthcare Ltd.
Both these vaccine candidates have undergone successful toxicity studies in rats, mice, and rabbits, and the data was submitted to the DCGI. Volunteers are to participate in the exercise for each of the two indigenously developed vaccine candidates.
In the first phase, a total of 375 volunteers will be participating, out of which, AIIMS Delhi will finalize 100 participants, aged between 18 and 55. The volunteers will be given a controlled dose to see if the vaccine is safe and how much of the dose can be administered. In the second phase, 750 people will be recruited from the age group between 12 and 65 years of age.

Since this was the first indigenous vaccine being developed by India, ICMR Director-General Dr Balram Bhargava in a letter to the principal investigators of the 12 sites, recently asked them to fast-track the human clinical trial approvals stating it is one of the “top priority projects which is being monitored at the top-most level of the government”.
In fact, India is one of the largest vaccine producers in the world, hence the fast-track vaccine development process will help break the chain of coronavirus transmission. The whole project is very crucial and is being closely administered.
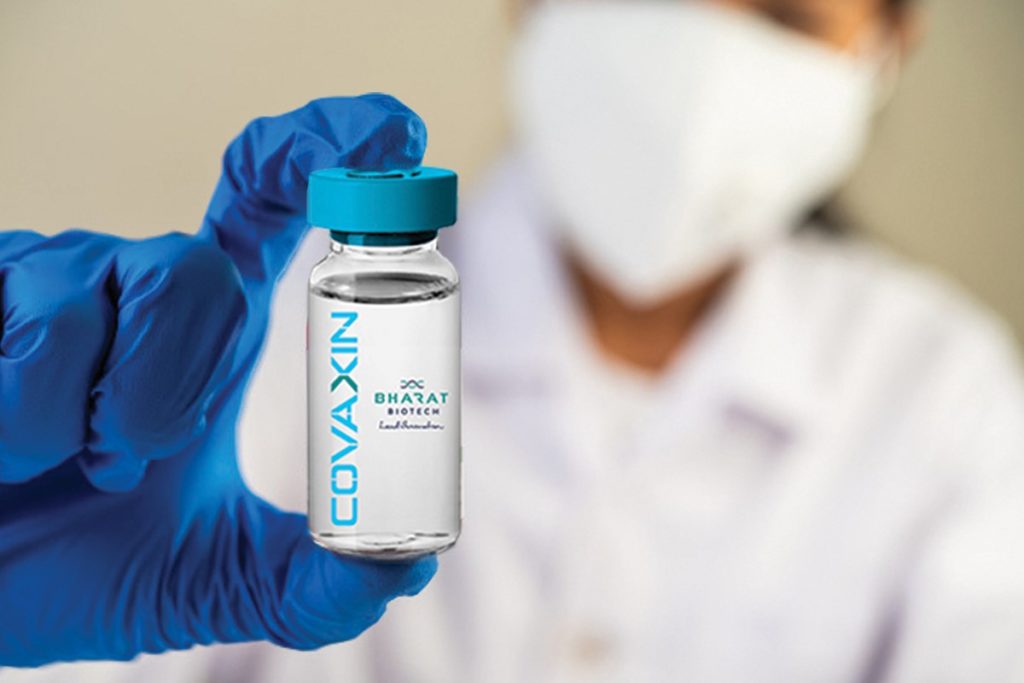
How you can participate in the human trials of COVAXIN
If one wishes to participate in human trials of Covaxin going on in AIIMS, the following things should be kept mind-
- The volunteers will have to undergo tests, which include the COVID-19 test.
- Volunteers should be healthy with no comorbid conditions and no history of COVID-19.
- A volunteer should be at least 18 years of age and less than 55 years of age to be eligible to participate.
- Pregnant women cannot participate in the trial in the first phase.
If found healthy sans comorbidities, they will be administered the vaccine. The clinical trial will be randomized, double-blind & placebo-controlled.
AIIMS has provided a dedicated email and phone number for anybody willing to participate in the trial. The volunteers need to register themselves for this project. Volunteers willing to become a part of the trials can send an email to [email protected] or SMS/call to 7428847499.
Further reading: